As of 1st May 2025, Epcoritamab is listed on the Pharmaceutical Benefit Scheme (PBS) making it more affordable for people with Refractory or Relapsed Diffuse Large B-cell Lymphoma.
Epcoritamab, (brand name EPKINLY®) is a new bispecific antibody treatment. It is now provisionally approved in Australia for people with Refractory or Relapsed Diffuse Large B-cell Lymphpoma (DLBCL). Provisional approval means the treatment has been approved based on early trials (phases 1 and 2) and can now be given to some patients.
Refractory means the lymphoma is not responding to treatment, it may be staying the same or getting worse even with treatment. Relapsed means the treatment initially worked well and you went into remission, but the lymphoma has come back.
Now, EPKINLY® is approved for some patients after just one prior treatment.
Researchers will continue to monitor its safety and effectiveness for ongoing approval. To be eligible for EPKINLY® you need to have already had two lines of treatment for your DLBCL.
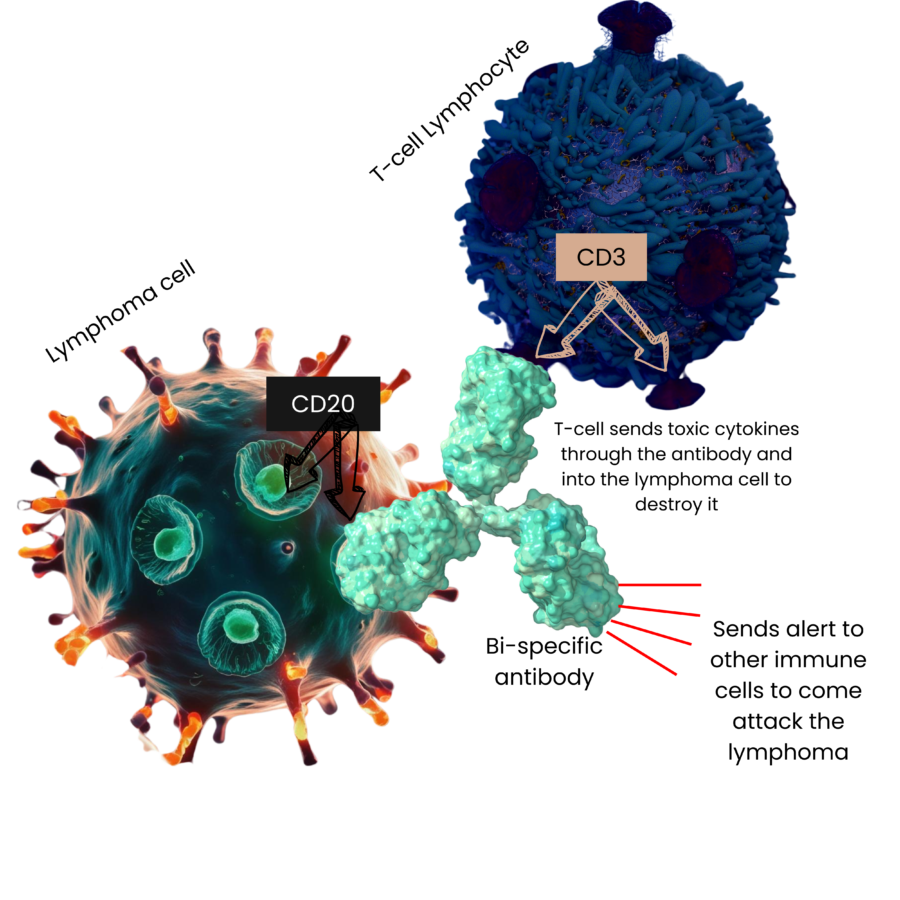
What are Bi-specific Antibodies?
Bispecific antibodies are medicines made from antibodies created in a laboratory that can attach to 2 different targets at the same time. It attaches to T-cells (a type of disease fighting immune cell) AND to lymphoma cells. This brings the T-cell close to the lymphoma and by doing this, it helps your immune system find and destroy the lymphoma.
Bi-specific antibodies help boost your body’s natural ability to fight DLBCL.
Access to Epcoritamab
Treasurer Jim Chalmers announced in the budget on 25th March that as of May 1st 2025, Epcoritamab will be listed on the Pharmaceutical Benefits Scheme (PBS), making it more affordable.
You can also check when EPKINLY® is listed on the PBS by visiting the Medicine Status Website here https://www.pbs.gov.au/medicinestatus/document/1248.html
Summary
- Epcoritamab is a new bi-specific antibody that helps your immune system fight lymphoma.
- Now provisionally approved by the TGA and available for some people with Refractory or Relapsed DLBCL.
- Will be more affordable to access once listed on the PBS – Planned for May 1st 2025.