Chimeric antigen receptor (CAR) T-cell therapy is a type of immunotherapy that uses your own immune system to destroy lymphoma cells.
On this page we will be discussing chimeric antigen receptor (CAR) T-cell therapy.
What does this mean?
Previously people with large B-cell lymphomas like DLBCL would need to have relapsed at least twice after treatment, or not respond to 2 different types of treatment before being able to access CAR T-Cell therapy. But now, eligible patients can access Gilead's CAR T-cell therapy - Yescarta after only one relapse or failed treatment.
This change means more people can benefit from CAR T-cell therapy earlier. Accessing this treatment earlier may improve outcomes for patients whose lymphoma has not responded to first-line treatment, or have had their DLBCL relapse after only one-line of treatment.
Overview
Understanding CAR T-cell therapy in lymphoma
Dr Koon Lee, Westmead Hospital.
Our immune system normally protects us and is the body’s defence against infection and disease, including cancer. It is made up of a network of organs and specialist white blood cells called lymphocytes. There are three types of lymphocytes that include:
- B lymphocytes (B-cells) – that make antibodies to fight infection
- T lymphocytes (T-cells) – help B-cells to make antibodies to identify infected cells, fight infection & directly kill infected or cancer cells in the body
- Natural killer (NK) cells – also attack cancer cells, infected cells and kill viruses
When lymphocytes gain certain genetic changes, they divide and grow uncontrollably resulting in lymphoma. This results in the immune system not being able to detect the abnormal cancerous cells or not being able to destroy them. Cancer cells can also develop ways to prevent the immune system from attacking them. For example, some cancer cells make special proteins on their surface that tell T-cells not to attack them.
Chemotherapy and radiation therapy have been the traditional ways to treat cancer. Immunotherapy is a type of treatment that improves the body’s ability to detect and attack cancer cells by using the body’s immune system.
It is an active area of clinical research and there are proven immunotherapy treatments. These include monoclonal antibody therapy (rituximab or obinutuzumab), other targeted therapies (eg. Pembrolizumab in Hodgkin lymphoma and primary mediastinal B-cell lymphoma), and most recently chimeric antigen receptor (CAR) T-cell therapy.
What is CAR T-cell therapy?
CAR T-cell therapy is a new type of immunotherapy that uses a patient’s own T-cells to recognise and attack cancer cells. CAR T-cell therapy uses specially altered T-cells to directly and precisely target certain cancers, including some subtypes of B-cell lymphoma. The reprogrammed T-cells strengthen the immune system to attack and kill the lymphoma cells.
A fraction of a patient’s own T-cells are collected from the blood using a procedure called apheresis. These cells are genetically re-engineered in a special laboratory, so they now carry special structures called chimeric antigen receptors (CAR) on their surface. CARs are proteins that are designed to attach to a specific target on cancer cells. For the currently approved products, that protein is called CD19 which is found on the surface of normal and cancerous B-cells.
The manufactured CAR T-cells are then re-infused into the patient (like a blood transfusion). When they bind to their target receptor, they multiply rapidly, and kill the target cells which in this case is the B-cell lymphoma and normal B lymphocytes. They continue to multiply and attack the cancer cells until they are all gone.
In some cases, it is thought that the CAR T-cells go on living in the body (called “persistence”) and can continue to keep the lymphoma or leukaemia at bay. This is why many think of CAR T-cells as a ‘living drug’.
Who is eligible for CAR T-cell therapy?
CAR T-cell therapy is publicly funded in Australia for people who meet the strict eligibility criteria that will be followed by an expert medical panel. Patients who have been diagnosed with one of the listed B-cell diseases, who have relapsed after at least 2 prior therapies or are refractory (have not responded to chemotherapy) and are medically fit, may be eligible for CAR T-cell therapy. CAR T-cell therapy can have serious side effects and is not suitable for everyone.
The majority of patients usually go into remission after receiving current standard first-line therapy that usually includes chemotherapy and a monoclonal antibody. CAR T-cell therapy is very expensive and costs over $500,000 per patient. The high cost is due to the specialist manufacturing process that is involved to create CAR T-cells. Only certain cancer centres will be specially trained to infuse CAR T-cell therapy and manage patient care.
The following lymphoma subtypes may be eligible:
- Diffuse Large B-cell Lymphoma
- Transformed Follicular Lymphoma
- Grade 3b Follicular Lymphoma
- Primary Mediastinal B-cell Lymphoma
- B-cell Acute Lymphoblastic Lymphoma (B-ALL) for people younger than 26
- Mantle Cell Lymphoma.
CAR T-cell therapy in Australia
In Australia, there have been two products that have had a positive recommendation from the Medical Services Advisory Committee (MSAC) and will both be soon be publicly funded. These products include:
- KymriahTM (tisagenlecleucel) a Novartis product and is publicly funded in Australia
- YescartaTM (axicabtagene ciloleucel) a Gilead product and is publicly funded in Australia
- TecartusTM (brexucabtagene autoeucel) a Gilead product which is publicly funded in Australia.
All referrals are discussed by medical experts at a national weekly CAR T-cell meeting. For more information speak to your haematologist or Lymphoma Australia.
Where can I have CAR T-cell therapy?
Adults |
Children |
|
Western Australia Fiona Stanley Hospital New South Wales Royal Prince Alfred Hospital Westmead Hospital Victoria Peter MacCallum Cancer Centre The Alfred Hospital Queensland Royal Brisbane and Women’s Hospital Townsville University Hospital Princess Alexandra Hospital |
Queensland Queensland Children’s hospital New South Wales Sydney Children’s Hospital Victoria Royal Children’s Hospital The Alfred Hospital |
Some hospitals not listed above may provide CAR T-cell treatment as part of a clinical trial. Ask your doctor if it is available where you are having treatment.
The CAR T-cell process
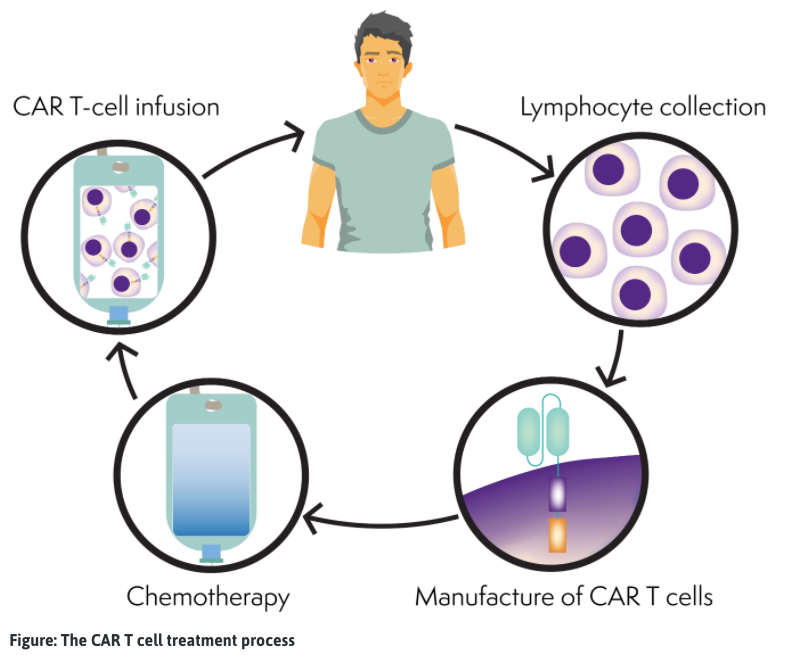
CAR T-cells are made individually for each person. You may receive other treatments, such as chemotherapy (bridging therapy), to keep your lymphoma under control while the CAR T-cells are being made (3-6 weeks).
- T-cell collection: Blood is taken from the patient. The white blood cells, that include T-cells, are separated out and the rest of the blood is put back into the patient’s bloodstream via apheresis (similar to collecting stem cells). The patient’s T-cells are sent to the lab for manufacturing.
- Manufacture of CAR T-cells: The T-cells are modified or genetically engineered (changed) so they can find and kill cancer cells. The engineered T-cells are now called CAR T-cells. The patient’s CAR T-cells are multiplied until there are millions of them and then are frozen. The CAR T-cells are then sent back to the patient’s hospital. This process can take several weeks.
- Chemotherapy: The patient will receive chemotherapy (lymphodepletion), to reduce the number of normal T-cells in the body to make space for the CAR T-cells, so they can expand (multiply) once administered. Typically, this chemotherapy is fludarabine and cyclophosphamide.
- CAR T-cell infusion: The patient’s CAR T-cells are thawed and then put back into the patient’s bloodstream, similar to receiving a blood transfusion or stem cells.
- In the patient’s body: The CAR T-cells multiply rapidly in the patient’s bloodstream. The CAR T-cell finds and kills the lymphoma cells. The CAR T-cells may remain in the bloodstream to attack if lymphoma returns.
- Recovery: The patient will be monitored carefully during and after the treatment. Patients who receive CAR T-cell therapy have a recovery period of approximately 2-3 months. During this period, patients will be evaluated for side effects and treatment response. During at least the first 30 days after discharge from hospital, patients need to remain close (within 20 min) to their treating hospital for regular follow up or urgent care if required.
Watch video - An introduction to CAR T-cell therapy
Possible side effects of CAR T-cell therapy
All medicines and cancer treatments can cause side effects. CAR T-cell therapy is a new type of treatment, and as researchers understand the treatment better, so are the management of these side effects. CAR T-cell therapy can cause serious side effects and the treatment is only given in hospitals with the facilities and specialist staff to manage these side effects effectively.
It is important to note that not all patients are going to be able to tolerate some of the possible side effects and therefore each patient’s medical and health status need careful consideration prior to undertaking CAR T-cell therapy.
Some of the common side effects may affect a significant proportion of patients and can lead to prolonged hospitalisation. The frequency of these side effects may be linked to the product used, and to patient and disease-related factors. These include:
- Cytokine release syndrome
- Fever and chills
- Low blood pressure and low oxygen levels
- Nervous system problems including; brain problems (encephalopathy), headache, twitching or shaking (tremor) or dizziness
- Rapid heart rate (tachycardia) and changes in heart rhythm (arrhythmia)
- Fatigue (extreme tiredness)
- Cough
- Digestive symptoms; nausea, vomiting, reduced appetite, diarrhoea and constipation
- Febrile neutropenia (low neutrophils – immune system) and infections
What is cytokine release syndrome (CRS)?
Cytokine release syndrome (CRS) is a potentially serious side effect and is associated with CAR T-cell therapy. Cytokines are chemical messengers that help the T-cells carry out their functions, that are produced when the CAR T-cells multiply in the body and kill the cancer cells. CRS symptoms can range from mild flu like symptoms through to more serious symptoms.
T-cells are designed to release cytokines (chemical messengers), that help to stimulate and direct the immune response. In the case of CRS, there is a rapid and massive release of cytokines into the bloodstream, which can cause dangerously high fevers and lower blood pressure. This can also be known as a ‘cytokine storm’.
Symptoms of cytokine release syndrome
CRS tends to arise within 1 to 5 days after the CAR T-cells are re-infused into the patient, although it may occur weeks later in some cases. For most patients, the condition is mild enough that it can be managed with supportive therapy and monitoring.
Signs and symptoms can include:
- Fever
- Fatigue
- Loss of appetite
- Muscle and joint pain
- Nausea and vomiting
- Diarrhoea
- Rashes
- Fast breathing
- Rapid heart rate
- Low blood pressure
- Seizures
- Headache
- Confusion or delirium
- Hallucinations
- Tremor
- Loss of coordination
Treatment of cytokine release syndrome
For many patients, CRS can be managed with standard supportive therapies such as steroids or intravenous fluids. As researchers have gained more experience with CAR T-cell therapy, they are learning how to better manage the more serious cases of CRS.
A standard therapy for patients to manage severe CRS is by administering a drug called tocilizumab (ActemraTM). This is a previously known drug to treat other inflammatory conditions, that is used to block a cytokine called IL-6. IL-6 is a cytokine that is secreted in high levels by T-cells in response to inflammation.
Some patients need to be admitted for the management of side effects and can be in hospital for a week or so. Some patients need to be admitted for additional support in the intensive care unit (ICU).
Nervous system problems
Many people treated with CAR T-cell therapy can experience nervous system problems within a few days of treatment, although problems can develop up to 8 weeks after treatment. Nervous system problems are usually mild and get better over a couple of weeks.
The most common problems that develop can affect the way that your brain works, where symptoms may include tremors, headaches, confusion, loss of balance, trouble speaking, seizures and sometimes hallucinations. These side effects generally subside after a few days, although for some can last for weeks.
Recovery of CAR T-cell therapy
Recovery can take time as the patient’s immune system recovers. The acute recovery period and close monitoring is typically 30 days after the CAR T-cell infusion. During this time patients must remain within 20 minutes of the treating cancer centre. They must also have a caregiver with them at all times to monitor for signs of fever, infection and neurologic difficulties. Most patients feel tired and don’t have much appetite during this period.
Immune system side effects
As CAR T-cell therapy affects your immune system, you might be at greater risk of infection, including serious infections after treatment. Your white blood cells might be low, and some people have very low B-cell levels and low antibody levels (antibodies are proteins that B-cells produce to help you fight infection). These problems can make it difficult for your body to fight infections. You may be given medication to help prevent infections. If you have low antibody levels, you may need immunoglobulin replacement therapy (infusion of antibodies) to help boost your immune system.
Clinical trials in Australia
There are many clinical trials that are currently being conducted around the world for a number of different blood cancers and solid tumour cancers. It has been shown to be most successful in certain B-cell lymphomas. There are currently clinical trials for B-cell lymphoma across Australia available (from first-line treatment) for:
- Diffuse large B-cell lymphoma
- Follicular lymphoma
- Mantle cell lymphoma
- B-cell non-Hodgkin lymphoma
- Chronic Lymphocytic leukaemia
For more information see ‘Understanding Clinical Trials’ webpage or see www.clinicaltrials.gov
International clinical trials
There are many clinical trials for CAR T-cell therapy around the world. The leading countries in development and clinical trials are located in the USA and Europe. There are clinical trials looking at many different lymphomas and leukemias from front line therapy, and in the relapsed or refractory setting.
Clinical trials for CAR T-cell therapy in humans started in 2012. It was only approved by the FDA (Food and Drug Administration in the USA) in 2017 that has since seen rapid global progress in the use of CAR T-cell therapy.
Researchers are still trying to understand how this therapy works, improving side effects and improving outcomes for patients. It is a rapidly developing area of research and exciting how far it has come in a short time.
For more information see ‘Understanding Clinical Trials’ webpage or see www.clinicaltrials.gov
For further information
- Speak to your haematologist about whether you are eligible or appropriate to have CAR T-cell therapy. If so, your haematologist can arrange a referral.
- For any queries related to patient eligibility for CAR T-cell therapy or how patients can access this treatment, please email: CAR-T.enquiry@petermac.org
- You can contact the Lymphoma Nurse Support Line: T 1800 953 081 or email: nurse@lymphoma.org.au for further information or advice.
Recorded presentations, expert interviews and resources
Patient pathway and CAR T-cell logistics – Education Session held 13th May 2025
Clinical Nurse Consultant (CNC) Karen Maddock from Westmead Hospital
Novel therapies in aggressive lymphoma & CAR T-cell therapy
Dr Michael Dickinson, Peter MacCallum Cancer Centre
CAR T-cell therapies and what it means for patients
A collaboration by Lymphoma Coalition and the Acute Leukemia Advocates Network – 30th June 2022
American Society of Hematology (ASH) expert interviews
European Hematology Association expert interviews
Some questions to ask your doctor
Am I eligible for CAR T-cell therapy?
Are there CAR T-cell therapy clinical trials available in Australia that I may be eligible for?
Are there any other treatments that are better for me?
Are there any other clinical trials available for me?
This page was last updated August 2020
Patient and family guide to CAR T-cell therapy - The patient experience
The below video “Patient and family guide to CAR T-cell therapy” was developed by the NSW government. Due to their privacy settings we cannot play it on our webpage, but if you click on the blue button “Watch on Vimeo“ you can access this video for free.


